 |
Basic Indoor Tanning Certification Course |
Chapter 2
Understanding Ultraviolet Radiation
- Electromagnetic spectrum
- UVA, UVB and UVC
- Wave theory
- Quantum theory
In order to truly understand the tanning process of the skin you
need to have at least a basic understanding of the properties and function
of light. Although light has played a central role in the histories
of religion, art and science and is so common to our everyday existence,
it can actually be quite elusive.
Understanding Ultraviolet Radiation
To understand ultraviolet radiation (UV) one needs to know UV's
placement in the electromagnetic spectrum. Ultraviolet light is located
between X-radiation and visible light. UV has a higher frequency and
shorter wavelength than visible light, and it has a lower frequency
and longer wavelength than X-radiation. UV with its longer wavelength
and less energy is less penetrating than X-ray and is sometimes absorbed
by matter. Photobiology studies the interaction of nonionizing radiation
between the electromagnetic spectrum and biologic systems. Nonionizing
radiation represents the ultraviolet, visible and near infrared regions
of the spectrum. Tanning occurs as a result of exposure to ultraviolet
radiation. To fully understand this reaction, you must familiarize yourself
with the electromagnetic spectrum.
Electromagnetic Spectrum
The electromagnetic spectrum is a way of visualizing the frequency
and wavelength proportions of different forms of energy. Electromagnetic
radiation has properties of both waves and particles. We divide the
electromagnetic spectrum in the UV range for medical purposes.
UVA is found in the region between 320 and 400 nm and is the
least powerful wavelength band of UV radiation. UVA acts primarily to
cause the melanin pigments in the skin to oxidize (darken) creating
the cosmetic tan and has limited power to cause erythema.
UVB is found in the region between 280 and 320 nm. It comprises
the wavelengths primarily associated with erythema (sunburn), is also
necessary for the production of vitamin D in the skin and is primarily
responsible for stimulating increased melanin production. UVB wavelengths
(at 305 nm) have 1,000 times more erythemal power than UVA wavelengths.
UVC is found in the region between approximately 200-280 nm
and is called germicidal UV because of its proven effectiveness in killing
sing-cell organisms. Solar radiation in the UVC range is absorbed almost
entirely by the atmosphere and that is fortunate considering that even
a short overexposure to UVC is very harmful to the eyes and causes severe
erythema (sunburn). One place where radiation in the UVC range can be
found is in the arc of a welding torch. For that reason, optical damage
referred to as "welders eye" is caused by UVC light. It should be noted
that UVC wavelengths are not produced by the UVR sources utilized by
the indoor tanning industry.
Wave Theory
Ultraviolet rays are similar to X-rays, white (visible) light, infrared
and other similar types of radiant energy. They are all electromagnetic
waves, wavelike disturbances associated with vibrating electric charges.
Most waves are transmitted by some medium; for example, you have all
seen waves on the surface of the water, in which case the water is the
transmitting material. When a stringed instrument is plucked, waves
are set up in the string, so the string becomes the transmitting material.
Strangely enough, no one knows what transmits electromagnetic waves,
however, we have proof that they are in fact transmitted.
Electromagnetic waves all travel at the same constant speed as light,
186,000 miles per second in a vacuum. All electromagnetic waves have
the same form and travel at the same speed, but differ in wavelength.
Wavelength is the distance between two successive crests in the wave.
The number of crests or cycles per second is the frequency of the wave.
The unit of frequency is hertz or 1 cycle per second. Therefore, if
the wavelength is decreased, then the frequency is increased. Frequency
and wavelength have an inverse relationship which is calculated with
one of two equations: (see illustration)
Electromagnetic Spectrum
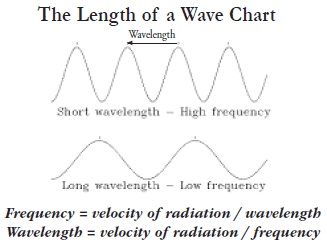 Frequency
= velocity of radiation / wavelength Frequency
= velocity of radiation / wavelength
or
Wavelength = velocity of radiation / frequency
Where the velocity of radiation is 186,000 miles per second. Frequency
is calculated using cycles per second and wavelength is calculated in
meters. The wavelengths of electromagnetic radiation vary in size from
a fraction of an angstrom unit (an angstrom is equal to ten billionths
of a meter) to thousands of meters, commonly called the "electromagnetic
spectrum." Some of the wavelengths of electromagnetic radiation from
this spectrum are classified as follows:
| SPECTRUM |
APPROXIMATE WAVELENGTH
|
| X-Ray |
0.1 - 100 angstroms |
| Vacuum |
10 - 200 nanometers |
| Ultraviolet C (UVC) |
200 - 290 nanometers |
| Ultraviolet B (UVB) |
290 - 320 nanometers |
| Ultraviolet A (UVA) |
320 - 400 nanometers |
| Visible light |
400 - 700 nanometers |
| Near Infrared |
0.74 - 1.5 micrometers |
| Middle Infrared |
1.5 - 5.6 micrometers |
| Far Infrared |
5.6 - 1,000 micrometer |
| Microwave/Radiowaves |
greater than one millimeter |
Therefore, the useful unit of measure for our purposes is the nanometer.
Radiations shorter than 10 nanometers (i.e. gamma rays or X-rays) generally
ionize molecules (remove electrons) producing positively or negatively
charged ions and are, therefore, known as ionizing radiation. Ultraviolet
radiation is absorbed by molecules and is known as nonionizing radiation.
Quantum (Particle) Theory
Another theory used in reference to the electromagnetic spectrum
is the quantum theory. In order to explain energy transfer, a bit of
energy called a photon was theorized. Photons have no mass and when
absorbed this energy is passed on to the absorbing molecule (such as
skin cells) and the photon no longer exists in its same state. The amount
of energy in a photon is directly proportional to the frequency of the
radiation. The energy of a photon increases as the frequency increases.
The more cycles per second (frequency) of any given photon, the more
energy the photon has. The energy of any given photon decreases as the
wavelength increases. The longer the wavelength, the less the frequency.
Light energy is expressed differently. We often express radiant energy
in terms of watts per square meter or milliwatts per square centimeter.
Skin exposure is usually expressed in joules per square centimeter.
A Joule, is a unit of measurement and is equivalent to the electrical
work done in one second by an electrical current of one ampere through
the resistance of one ohm; named for its inventor, British Physicist,
J.P. Joule (1818-1889).
|